European Medicines Verification Organisation (EMVO)
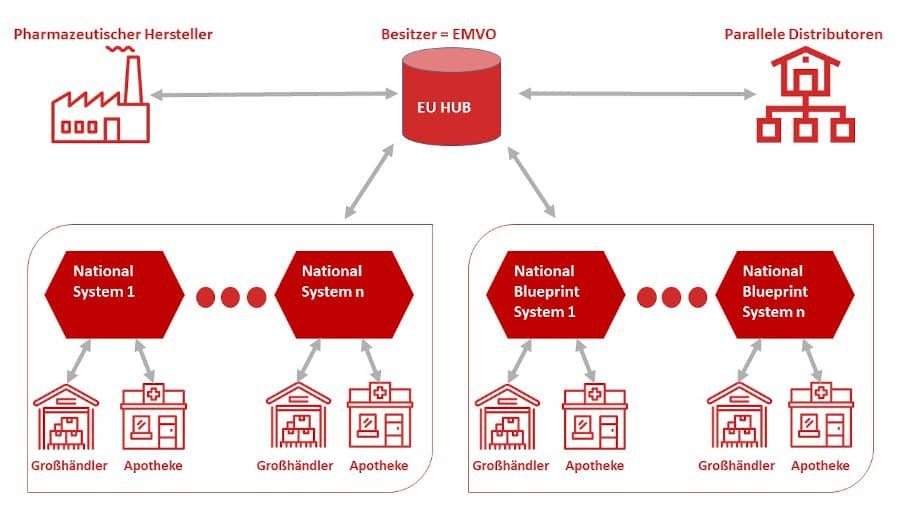
The European Medicines (Verification Organisation (EMVO) was founded in 2015 and is based in Brussels. The reason for the foundation was the introduction of Directive 2011/62/EU (Falsified Medicines Directive (FMD)) and the Delegated Regulation (EU/2016/161). The EMVO oversees the European Medicines Verification System (EMVS). The directive aims to protect patients from falsified medicines and concerns manufacturers,Pharmaceutical wholesalerand distributors (pharmacies/hospitals).
EMVO consists of several founding members. The founding members are:
EFPIA (the European Federation of Pharmaceutical Industries and Associations),
Medicines for Europe (the European Generic and Biosimilar MedicinesAssociation),
PGEU (the Pharmaceutical Group of the European Union),
GIRP (the European Healthcare Distribution Association)
EAEPC (the European Association of Euro-Pharmaceutical Companies)
HOPE (European Hospital and Healthcare Federation)
EAHP (European Association of Hospital Pharmacists)
The members have different financial tasks in EMVO. Contributions from wholesalers and pharmacies are used to finance EMVO and national system. Pharmaceutical manufacturers are responsible for financing the technical development of theEMVS responsible. No public funds are needed to ensure the safety of the drug chain
EMVO is the owner of the EU Hub. TheEU Hub is the hub of all drug movements. All info is stored in the database.