EMVS - European Medicines Verification System
The European Medicines Verification System (EMVS) plays an important role in the EU Regulation 2011/62/EU.
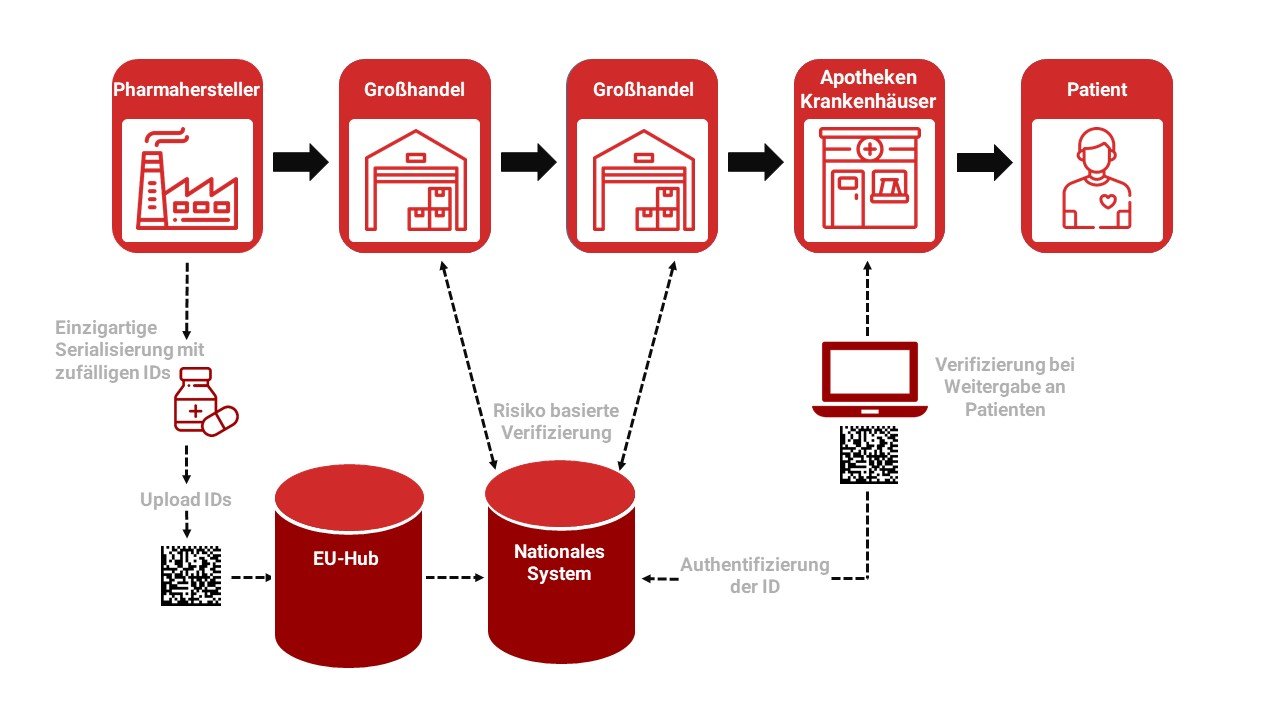
The European Medicines Verification System EMVS consists of components:
European HUB
Repositories
And the infrastructure to connect the EU hub with national system
Manufacturers and parallel importers/distributors are networked via the EU Hub. And Repositories networks pharmaceutical dispensaries with wholesalers.
EMVS is funded by the pharmaceutical industry itself, with no involvement from governments or the public.