Medical Device Regulation MDR
The new EU Regulation (EU) 2017/746 and EU Regulation (EU) 2017/745 present new challenges for participants in the pharma supply chain.
Medical Device Regulation (MDR)
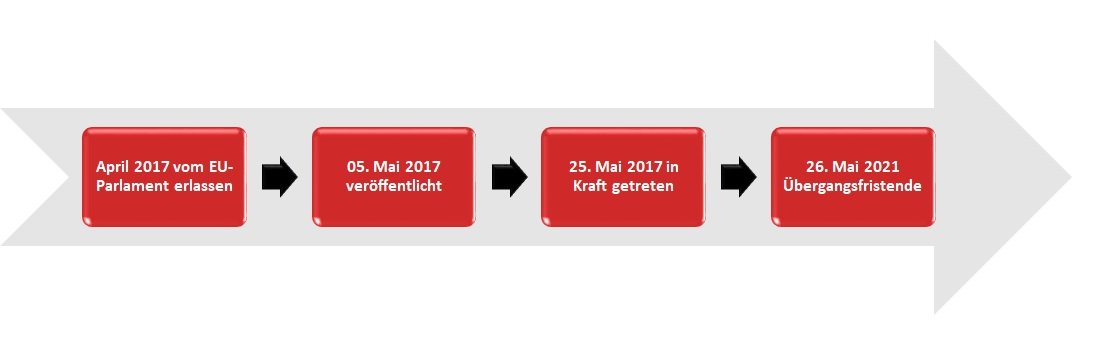
The Medical Device Regulation (MDR) was adopted by the European Parliament in April 2017. It was published on 05.05.2017 and came into force on 25.05.2017. The MDR has a transition period until 26.05.2021.
What is the Medical Device Regulation MDR?
The Medical Device Regulation (MDR) is a regulation issued by the European Parliament to ensure the safety and high standard of medical devices. Throughout the supply chain, products must be monitored and scanned.
For this purpose, the products are provided with a Unique Device Identifier (UDI). In the future, the scanned code together with additional reportable information must be reported to the European database of medical devices (EUDAMED).
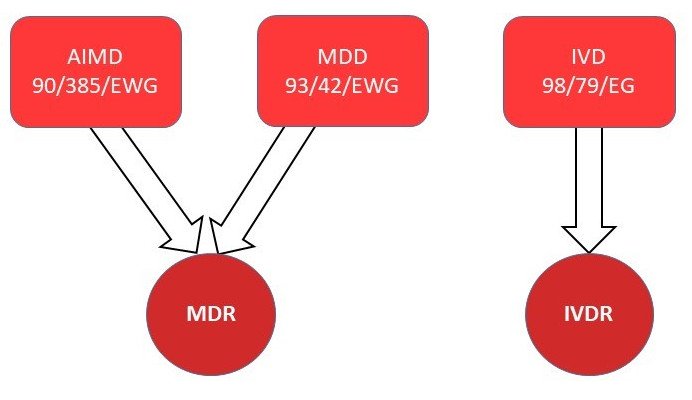
The Medical Device Regulation supersedes the following two directives:
Medical Device Directive (MDD) 93/42 EEC
Active Implantable Medical Device (AIMD) 90/385 EWG
What are the objectives of the Medical Device Regulation:
The regulation is intended to ensure a smoothly functioning internal market for medical devices" Above all, small and medium-sized enterprises are to be taken into account"
The regulation also sets high standards for the quality and safety of medical devices"
The two goals are pursued in parallel, they are inseparable and of absolutely equal importance"
Exceptions
The regulation also contains exceptions, because not all products have to be scanned" The exception concerns the products IIa to III, if a certificate was issued before the start of validity" The certificate may be valid for a maximum of 4 years after the start of validity" This transition period is called "soft transition"" The intended purpose or design features may not be changed, otherwise the certificate will expire"
"What is considered a medical device"
"Medical device" means an instrument, apparatus, device, software, implant, reagent, material, or other item that is intended for human use according to the manufacturer" These devices are intended to save lives, help heal, and improve the quality of people´s lives" The term is defined in Article 2(1) of Regulation (EU) 2017/745"
Depending on the classification of the medical device, the EU regulation on MDR takes effect on the timeline and requires a process change or the reporting obligations:
Class 3: from 2022, Class 2: from 2023, Class 1: from 2025
Healthcare devices at COSYS
Compliance with MDR and IVDR starts with the right hardware. Only professional barcode scanning hardware ensures compliance with EU regulations and allows seamless traceability of medical devices and in vitro diagnostics along the supply chain. By scanning barcodes and data matrix codes, traceability is made possible. Therefore, MDE devices and handheld scanners are of high importance for pharmaceutical companies to meet the requirements of the EU.
With COSYS you have a competent partner who can advise you in detail about the right hardware. In addition to classic MDE devices for harsh working conditions, we also offer you MDE hardware specially suited for the healthcare sector, so that you can meet EU requirements.
Our experts will be happy to take the time to find a perfect hardware solution for you!
In addition to hardware solutions, the camera can also be used to capture barcodes and data matrix codes. The COSYS Performance Scanning software captures codes at lightning speed, and the smartphone camera can be used to scan the codes.
Turn your smartphones, tablets and wearables into true enterprise-class barcode scanners that can be used in any business environment with COSYS. Image recognition algorithms and the high processing power of smartphones allow codes to be scanned at breathtaking speed and accuracy - significantly faster and better than ordinary barcode scanners.