Falsified Medicines Directive (FMD)
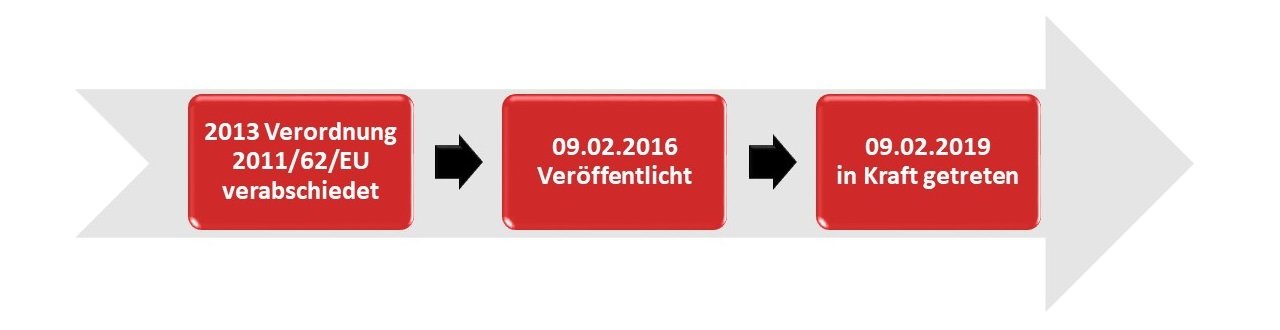
This directive was adopted in 2013 and published on 09.02.2016. The Falsified Medicines Directive entered into force on 09.02.2019.
What is Falsified Medicines Directive (FMD)?
The risk of drug counterfeiting is increasing worldwide. The drugs may contain wrong or incorrectly dosed active ingredients. The counterfeit drug trade quickly brings a high profit and the Internet makes it difficult to trace. In addition, the risk increases further due to global transportation routes.
As a result, the European Union has established a set of measures in Directive 2011/62/EU to prevent counterfeit medicines from entering the legal supply chain.
The drugs must be protected against counterfeiting and clearly identified. This is done by means of a unique identifier (2D DataMatrix code). Manufacturers must provide a product identifier, serial number, lot and batch number, and expiration date.
Medicines are scanned along the entire supply chain and the data is transferred to the national database (EU hub).
What are the objectives of the Falsified Medicines Directive:
The aim of the Falsified Medicines Directive is to protect the health and safety of medicines.
Exceptions
Only products for human use must be provided with a code. Test products can continue to be shipped without being recorded.
Procedure for pharmacies:
Pharmacies scan the medications, the data is sent to the national database, verified, and then the pharmacy receives a message about the product, whether it is genuine or counterfeit.
Procedure for wholesalers:
Wholesalers must scan medications when they purchase these medications from
the original manufacturer,
Wholesalers acting as marketing authorization holders,
and wholesalers who have a contract with MAH.
In addition, the drugs must be scanned when they are returned from pharmacies.
Healthcare devices at COSYS
Compliance with MDR and IVDR starts with the right hardware. Only professional barcode scanning hardware ensures compliance with EU regulations and allows seamless traceability of medical devices and in vitro diagnostics along the supply chain. By scanning barcodes and data matrix codes, traceability is made possible. Therefore, MDE devices and handheld scanners are of high importance for pharmaceutical companies to meet the requirements of the EU.
With COSYS you have a competent partner who can advise you in detail about the right hardware. In addition to classic MDE devices for harsh working conditions, we also offer you MDE hardware specially suited for the healthcare sector, so that you can meet EU requirements.
Our experts will be happy to take the time to find a perfect hardware solution for you!
In addition to hardware solutions, the camera can also be used to capture barcodes and data matrix codes. The COSYS Performance Scanning software captures codes at lightning speed, and the smartphone camera can be used to scan the codes.
Turn your smartphones, tablets and wearables into true enterprise-class barcode scanners that can be used in any business environment with COSYS. Image recognition algorithms and the high processing power of smartphones allow codes to be scanned at breathtaking speed and accuracy - significantly faster and better than ordinary barcode scanners.