EU Pharma Regulation MDR / IVDR / FMD
The three EU regulations (medical devices (MDR), in vitro diagnostics (IVDR) and anti-counterfeiting (FMD)) pose major changes for the pharmaceutical trade. All pharmaceuticals and medical devices must be traceable throughout the entire supply chain. COSYS supports you with the Track and Trace solution, which enables a seamless tracking system using the most innovative technology.Medical Device Regulation & In Vitro Diagnostics Solution
The newPharma EU Regulation (EU) 2017/746 and EU Regulation (EU)2017/745 pose new challenges for participants in the pharma supply chain.
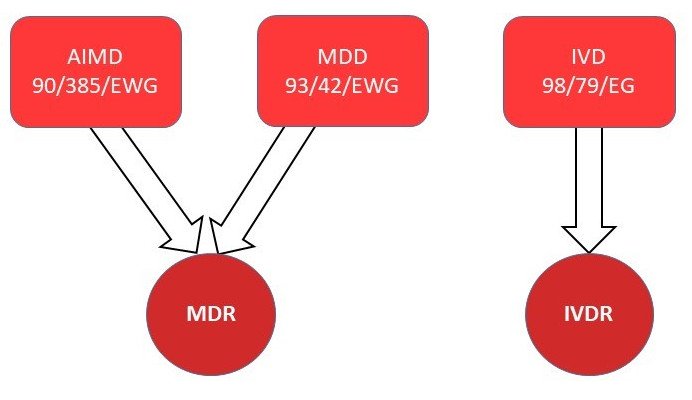
According to the regulations, all medical devices and in-vitro diagnostics must be provided with a UDI code (Unique Device Identifier). Manufacturers receive this code from the EU database (Eudamed). The products are then scanned along the entire delivery route and also reported to Eudamed.
COSYS has a solution for these EU regulations for the implementation of the directives. From scanning medical devices with the unique product identification number (UDI) to reporting to the medical device verification system, COSYS can deliver a complete system.
Tracking of all pharmaceutical products
Available as stand alone or complete solution in other COSYS software
Quick control in case of recall
Falsified Medicines Directive FMD Track & Trace solution
In February 2019, the EU Regulation 2011/62/EU came into force. The regulation states that medicinal products must be labeled with a 2D data matrix code. This code is composed of product identifier, a serial number, a lot and batch number and an expiry date. These codes are scanned from the manufacturer to the end customer and the data is reported to the EU Hub. With the help of COSYS Track & Trace solution you can easily implement regulation. The COSYS solution works from product registration, to scanning at the end user. Even a return is no problem.
Healthcare devices at COSYS
Compliance with MDR and IVDR starts with the right hardware. Only professional barcode scanning hardware ensures compliance with EU regulations and allows seamless traceability of medical devices and in vitro diagnostics along the supply chain. By scanning barcodes and data matrix codes, traceability is made possible. Therefore, MDE devices and handheld scanners are of high importance for pharmaceutical companies to meet the requirements of the EU.
With COSYS you have a competent partner who can advise you in detail about the right hardware. In addition to classic MDE devices for harsh working conditions, we also offer you MDE hardware specially suited for the healthcare sector, so that you can meet EU requirements.
Our experts will be happy to take the time to find a perfect hardware solution for you!
In addition to hardware solutions, the camera can also be used to capture barcodes and data matrix codes. The COSYS Performance Scanning software captures codes at lightning speed, and the smartphone camera can be used to scan the codes.
Turn your smartphones, tablets and wearables into true enterprise-class barcode scanners that can be used in any business environment with COSYS. Image recognition algorithms and the high processing power of smartphones allow codes to be scanned at breathtaking speed and accuracy - significantly faster and better than ordinary barcode scanners.